Name of Standard: National Standard of Electrolytic Conductivity in the range (0,005 - 10) S.m-1
Code designation: ECM 331-1/19-061
Year of promulgation: 2018
Department: 6016 ČMI OI Brno, Okružní 31, 638 00 Brno
Guarantor: Mgr. Martina Vičarová, Ph.D.
The number of CMC lines provided: 3
| Range of electrolytic conductivity (S.m-1) | Expanded uncertainty (k=2) (%) |
|---|---|
| (0.005-0.015) | (1.70-0.23) |
| (0.015-0.15) | (0.62-0.19) |
| (0.15-10) | (0.10-0.10) |
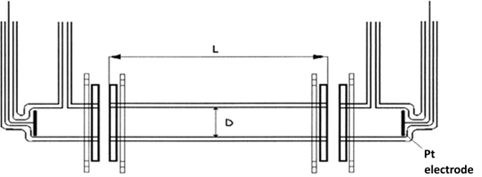
The basis of primary standard of electrolytic conductivity is glass conductometry cell made in the Czech Republic – in the Institute of Plasma Physics of Czech Academy of Sciences, workplace in Turnov, Optical Development workroom. Cell consists of three parts. In the two end parts there are platinum electrodes built-in, which are charged with alternating voltage. The middle part has got precisely measured length parameters (inner diameter D and length of cylinder L). Based on these length parameters the constant of middle part is calculated.
K= L/S = L/ (π D2/4)
where L is length of middle part of primary cell, calibrated, in m; S is the area of cross section of middle part of primary cell, in m2; D is diameter of middle part of primary cell, calibrated, in m.
Primary conductometry cell, schematic sketch
The principle of the standard is based on measurement of impedance of reference materials with different concentrations of potassium chloride in two positions – measurement with the middle part and measurement of the same solution without the middle part. Electrolytic conductivity κ is calculated from subtraction of impedances (Z₂ − Z₁), which are measured in these two positions of cell and ratio of length L of the middle part of cell to its area S (constant of cell K). Parasitic phenomenon emerging on platinum electrodes is being eliminated this way, because it contributes the same amount to conductance of cell both with and without the middle part.
R0 = Z2 - Z1 = ΔZ
K= L/S
κ = K/R0
where κ is electrolytic conductivity, in S∙m⁻¹; ∆Z is the change of impedance corresponding the change of length of cell ∆L, in Ω; K is constant of conductivity cell in m⁻¹, R₀ is resistance of column of electrolyte of length L, in Ω.
The Standard consists of the following parts: conductometry cell, bridge Agilent 4284A, resistance thermometer Pt 25 Tinsley 5187SA, transmitter Anton Paar MKT 50.
Auxiliary equipment: air thermostat TB4-LT, scales Mettler Toledo PR 5003 comparator and XP 56/M, scales Sartorius CP 225D-OCE, hygrometer with thermometer COMET D3121, pressure gauge Druck DPI 141, laboratory furnace 003 LP, computer with LabVIEW program.

